Centennial, Colo. — March 8, 2023 — AlloSource®, one of the largest allograft providers creating innovative cellular and tissue products to help surgeons heal their patients, today announced positive prospective clinical outcomes for the repair of focal articular cartilage defects in the knee using ProChondrix® CR, a readily available full thickness intact living cellular articular cartilage allograft.
The prospective clinical study recorded pre and postoperative International Knee Documentation Committee (IKDC – assesses overall patient function) and Knee Injury and Osteoarthritis Outcome Score scores (KOOS – assesses pain, functions in daily living and sports and quality of life). These patient-reported outcomes showed sustained positive result scores with statistical significance achieved at the 6-, 12-, 24- and 36- months postoperatively. Most notably at 36 months, IKDC scores improved by 86.3% (Figure 1) and KOOS Quality of Life scores improved by 132.5% from the preoperative scores (Figure 2).
“ProChondrix CR has demonstrated promising data in the laboratory. This clinical data continues to tell the story of the positive impact ProChondrix CR can have on patient recovery,” said Carolyn Rorick, AlloSource Senior Director Product Development, Innovation and Clinical Affairs. “The study is ongoing, so we will continue to gather additional data to confirm the longer-term results.”
ProChondrix CR is a living intact hyaline cartilage product that contains functional chrondrocytes and other biological components necessary for repair and replacement of damaged articular cartilage tissue.1 Created with AlloSource’s proprietary cartilage cryopreservation process, ViaTrue™, ProChondrix CR has an average of 94.97% chondrocyte viability after two years of cryopreserved storage. 2 The two-year shelf life helps to alleviate inventory management challenges and provides surgeons with more flexibility for their patients.
For additional detail on ProChondrix CR’s clinical outcomes visit booth #6627 at the American Academy of Orthopaedic Surgeons Annual Meeting in Las Vegas March 7-11, 2023 or allosource.org.
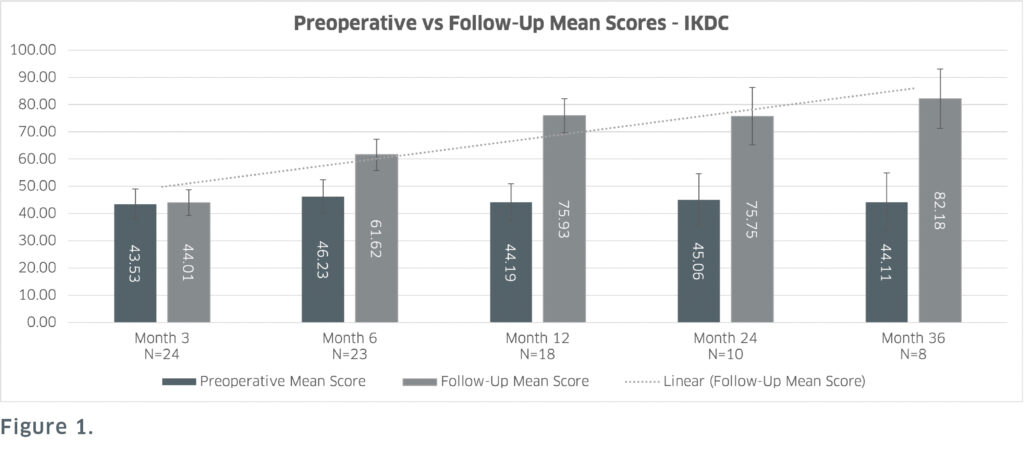
from preoperative scores to follow-up scores, except for the month 3 follow-up. There is also a trend of follow-up scores generally
improving at each subsequent timepoint which includes an 86.3% increase from preoperative scores at 36 months follow-up.
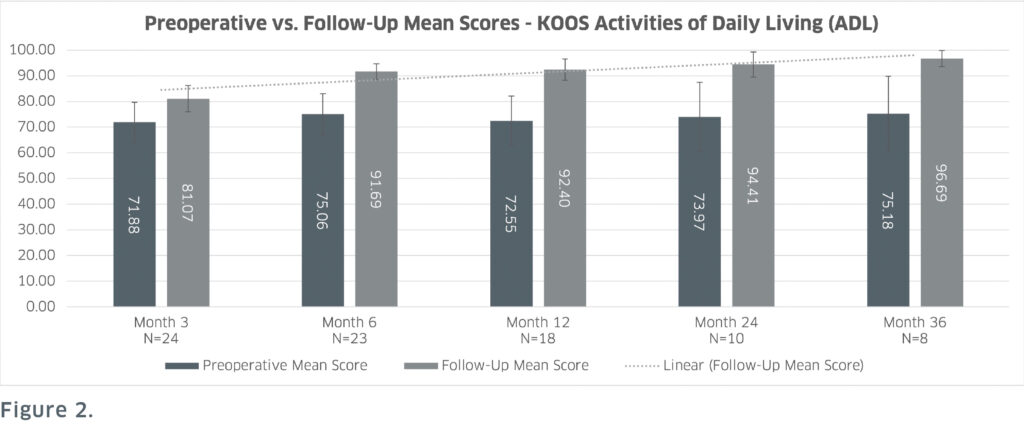
###
About AlloSource
AlloSource, one of the largest human tissue providers, honors tissue donors by creating innovative dermal, cartilage, tendon, fascia, bone, amniotic, and living cellular allografts to help heal patients. Since 1994, the Colorado-based nonprofit organization has continued to advance its allografts to improve patient outcomes, serving as a trusted tissue partner to the medical community. AlloSource® is registered with the FDA as a tissue establishment and accredited by the American Association of Tissue Banks. Learn more at allosource.org.
- Data on file.
- Rorick et al. Journal of Orthopaedic Surgery and Research – 2020.



